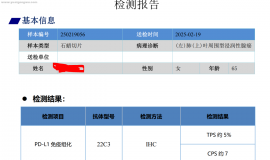
马дёҠжіЁеҶҢпјҢз»“дәӨжӣҙеӨҡеҘҪеҸӢпјҢдә«з”ЁжӣҙеӨҡеҠҹиғҪпјҢи®©дҪ иҪ»жқҫзҺ©иҪ¬зӨҫеҢәгҖӮ
жӮЁйңҖиҰҒ зҷ»еҪ• жүҚеҸҜд»ҘдёӢиҪҪжҲ–жҹҘзңӢпјҢжІЎжңүиҙҰеҸ·пјҹз«ӢеҚіжіЁеҶҢ

x
HydralazineдёҺvalproateпјҲдёҷжҲҠй…ёзӣҗпјүжҳҫ然еҸҜд»Ҙе…ӢжңҚеҢ–з–—жҠ—иҚҜжҖ§
еҗҲдҪөдёӨз§Қеёёз”ЁиҚҜзү©пјҡhydralazineдёҺmagnesium valproateпјҢжҳҫ然еҸҜд»Ҙе…ӢжңҚеҢ–з–—жҠ—иҚҜжҖ§гҖӮдёҖйЎ№еҸ‘иЎЁж–ј9жңҲеҸ·иӮҝзҳӨеӯҰ科еӯҰиӘҢдёӯзҡ„第дәҢжңҹдё–д»Јз ”з©¶з»“жһңжҳҫзӨәпјҢиҝҷж ·зҡ„иҚҜзү©еҗҲдҪөз–—жі•еҜ№15дҪҚз—…жӮЈдёӯзҡ„12дҪҚпјҲ80%пјүжңүдёҙеәҠеҘҪеӨ„пјӣиҝҷдәӣз—…жӮЈзҪ№жӮЈеҗ„з§ҚдёҚеҗҢзҷҢз—ҮпјҢдё”е„ҳз®ЎжҺҘеҸ—еҢ–еӯҰжІ»з–—пјҢз–ҫз—…д»Қ然иҝӣеұ•дёӯпјӣеңЁдҪҝз”ЁиҝҮhydralazineдёҺvalproateжІ»з–—еҗҺпјҢйҮҚж–°дҪҝз”ЁдёҖж ·зҡ„еҢ–еӯҰжІ»з–—еҗҺпјҢ4дҪҚз—…жӮЈеҮәзҺ°йғЁд»ҪеҸҚеә”пјҢиҖҢ8дҪҚз—…жӮЈз–ҫз—…зЁіе®ҡдёӢжқҘгҖӮ
гҖҖгҖҖ
гҖҖгҖҖиҝҷж ·зҡ„еҗҲдҪөз–—жі•иў«и®ӨзӮәйҖҸиҝҮйҖҶиҪ¬еҜјиҮҙеҢ–еӯҰз–—жі•жҠ—иҚҜжҖ§зҡ„иЎЁи§Ӯеҹәеӣ и„ұеәҸпјҡhydralazineпјҢжҳҜдёҖз§Қж—©жңҹзҡ„йҷҚиЎҖеҺӢиҚҜзү©пјҢд№ҹеҸҜд»ҘдҪңзӮәDNAз”ІеҹәеҢ–зҡ„жҠ‘еҲ¶еүӮпјҢ然иҖҢпјҢжҠ—зҷІзҷҮиҚҜзү©magnesium valproateжҳҜз»„з»ҮиӣӢзҷҪеҺ»д№ҷйҶҜеҢ–зҡ„дёҖз§ҚжҠ‘еҲ¶еүӮгҖӮ
гҖҖгҖҖ
гҖҖгҖҖдё»иҰҒдҪңиҖ…еўЁиҘҝе“ҘTlapanеёӮеӣҪ家зҷҢз—Үжңәжһ„Alfonso Duenas-GonzalezеҢ»еёҲеҗ‘MedscapeиЎЁзӨәпјҢиҝҷдәӣз»“жһңйқһеёёжё…жҘҡең°жҳҫзӨәпјҢз”ІеҹәеҢ–дёҺз»„з»ҮиӣӢзҷҪд№ҷйҶҜеҢ–дјҡжҹҗдёӘзЁӢеәҰдёҠең°ж¶ҲйҷӨиЎЁи§Ӯеҹәеӣ ж Үи®°пјҢйҖ жҲҗеҢ–еӯҰжІ»з–—зҡ„жҠ—иҚҜжҖ§пјҢе·Із»Ҹж–јеӨҡж¬Ўзҡ„е®һйӘҢжЁЎејҸдёӯиҺ·еҫ—иҜҒе®һпјӣд»–зҡ„еӣўйҳҹзӣ®еүҚжӯЈеңЁиҝӣиЎҢдёӨйЎ№йҡҸжңәеҲҶжҙҫ第дёүжңҹдёҙеәҠиҜ•йӘҢпјҢдҪҝз”ЁhydralazineдёҺvalproateпјҢд»–иЎЁзӨәпјҢеўЁиҘҝе“ҘеҚ«з”ҹеҪ“еұҖзӣ®еүҚжӯЈеңЁе®ЎжҹҘиҝҷж ·зҡ„еҗҲдҪөз–—жі•пјҲеҸҜиғҪд»ҘTranskripзҡ„е•Ҷе“ҒеҗҚдёҠеёӮпјүпјҢдҪҝз”Ёж–јеҜ№жІ»з–—еҸҚеә”дёҚдҪізҡ„еӣәдҪ“иӮҝзҳӨз—…жӮЈиә«дёҠгҖӮ
гҖҖгҖҖ
гҖҖгҖҖиә«зӮәдёҖдёӘиӮҝзҳӨеӯҰ家пјҢд»–иЎЁзӨәпјҢдҪҝз”Ёиҝҷж ·зҡ„еҗҲдҪөз–—жі•ж–јжІЎжңүе…¶д»–жІ»з–—йҖүжӢ©дё”е·Із»ҸдёҺз—…жӮЈжңүиҝҮж·ұе…Ҙи®Ёи®әзҡ„жІ»з–—еҸҚеә”дёҚдҪіз—…жӮЈпјҢжҳҜеҖјеҫ—иҖғиҷ‘зҡ„пјӣд»–зҡ„иҜ„и®әжҳҜпјҢиҝҷж ·зҡ„е»әи®®жҳҜеҹәж–јжӯӨдёӨз§ҚиҚҜзү©зҡ„е®үе…ЁжҖ§иүҜеҘҪпјӣ然иҖҢпјҢд»–йҷ„еёҰиЎЁзӨәпјҢеҸӘжңүеңЁз¬¬дёүжңҹдёҙеәҠз ”з©¶е®ҢжҲҗеҗҺпјҢжҲ‘们жүҚзҹҘйҒ“иҝҷж ·зҡ„жІ»з–—еңЁзҷҢз—Үз–—жі•дёҠзҡ„ең°дҪҚгҖӮ
гҖҖгҖҖ
гҖҖгҖҖеңЁж–Үз« йҡҸеҗҺзҡ„иҜ„и®әдёӯпјҢжҸҗеҲ°иҝҷж ·зҡ„з»“жһңжҳҜзӣёеҪ“еҲәжҝҖзҡ„пјҢдҪҶдё»зј–и®ӨзӮәиҝҷйЎ№з»“жһңеә”иҜҘи°Ёж…ҺйҳҗйҮҠпјҢеӣ зӮәдёҚеҗҢзҡ„иӮҝзҳӨдёҺеҢ–еӯҰжІ»з–—д№Ӣй—ҙе·®ејӮеҫҲеӨ§пјҢжҺҘеҸ—第дёҖйҖұзҡ„hydralazineдёҺmagnesium valproateжІ»з–—е°ұиҝӣеұ•зҡ„з—…жӮЈпјҢиў«жҺ’йҷӨж–јиҜ•йӘҢд№ӢеӨ–пјӣдё»зј–иӢұеӣҪдјҰж•ҰImperialеӨ§еӯҰRobert BrownеҚҡеЈ«д»ҘеҸҠиӢҸж је…°ж јжӢүж–Ҝе“ҘеҪјеҫ—жЈ®иҘҝиӢҸж је…°зҷҢз—ҮдёӯеҝғRosalind GlasspoolеҢ»еёҲжҢҮеҮәпјҢдёҚз®ЎеҰӮдҪ•пјҢд»ҘдёҖз§ҚзӣёеҜ№иҖҗеҸ—жҖ§иүҜеҘҪзҡ„жІ»з–—е°ұеҸҜд»ҘйҖҶиҪ¬еҢ–еӯҰжІ»з–—жҠ—иҚҜжҖ§жҳҜеҫҲеј”иҜЎзҡ„пјҢдё”иҝҷж ·зҡ„иЎЁи§Ӯеҹәеӣ жІ»з–—жӯЈеңЁиҝӣиЎҢи®ёеӨҡдёҙеәҠз ”з©¶дёӯгҖӮ
гҖҖгҖҖ
гҖҖгҖҖгҖҗиӮҝзҳӨдёҺеҢ–еӯҰжІ»з–—ж—¶зЁӢзҡ„е·®ејӮгҖ‘
гҖҖгҖҖзӣ®еүҚиҝҷ项第дәҢжңҹдёҙеәҠиҜ•йӘҢдёӯпјҢз ”з©¶иҖ…иЎЁзӨә27дҪҚз—…жӮЈзӯҫзҪІеҸ—иҜ•иҖ…еҗҢж„Ҹд№ҰпјҢдҪҶе…¶дёӯ3дҪҚдёҚз¬ҰеҗҲз ”з©¶ж”¶зәіж Үжә–пјҢ且并жңӘдҪҝз”ЁhydralazineдёҺvalproateпјҢ然иҖҢпјҢеҸҰеӨ–7дҪҚз—…жӮЈеңЁз¬¬дёҖйҖұгҖҒ第дёҖж¬ЎжҠ•дәҲиҚҜзү©еүҚе°ұеӣ зӮәз–ҫз—…иҝӣеұ•ж”ҫејғжІ»з–—пјӣжӣҙжңү2дҪҚз—…жӮЈеңЁиҜ•йӘҢи®ЎеҲ’дёӯе®ҢжҲҗдёӨдёӘйҖұжңҹзҡ„еҢ–еӯҰжІ»з–—пјҢдҪҶжҳҜж— жі•иҜ„дј°зҡ„пјҢеӣ зӮәж— жі•зЎ®и®Өз–ҫз—…иҝӣеұ•жҳҜеҗҰеҸ‘з”ҹ於他们зәіе…Ҙи®Ўз”»д№ӢеүҚпјӣиҝҷ2дҪҚз—…жӮЈзәіе…ҘжңҖеҗҺиҜ„дј°жҜ’жҖ§зҡ„17дҪҚжӮЈиҖ…дёӯпјҢдҪҶ并жңӘ收зәіеҲ°иҜ„дј°з–—ж•Ҳзҡ„15дҪҚжӮЈиҖ…дёӯгҖӮ
гҖҖгҖҖ
гҖҖгҖҖ收зәіз—…жӮЈзҡ„зҷҢз—ҮеҢ…жӢ¬еҚөе·ўпјҲ7дҪҚз—…жӮЈпјүгҖҒеӯҗе®«йўҲпјҲ3дҪҚпјүгҖҒд№ізҷҢпјҲ3дҪҚпјүгҖҒиӮәзҷҢпјҲ1дҪҚпјүгҖҒдёҺзқӘдёёзҷҢпјҲ1дҪҚпјүпјҢиҖҢдҪҝз”Ёзҡ„еҢ–еӯҰжІ»з–—еҢ…жӢ¬cisplatinгҖҒcarboplatinгҖҒpaclitaxelгҖҒvinorelbineгҖҒgemcitabineгҖҒpemetrexedгҖҒtopotecanгҖҒdoxorubicinгҖҒcyclophosphamideдёҺanstrazoleпјӣжүҖжңүз—…жӮЈиҮіе°‘жҺҘеҸ—дёӨдёӘйҖұжңҹзҡ„еҢ–еӯҰжІ»з–—пјҢдё”еңЁз¬¬дәҢдёӘжҲ–жҳҜ第дёүдёӘеҢ–еӯҰжІ»з–—з–—зЁӢй—ҙиҝӣеұ•пјӣзЎ®и®Өз–ҫз—…иҝӣеұ•еҗҺзҡ„30еӨ©еҶ…ејҖе§Ӣи®ЎеҲ’жІ»з–—пјҢеңЁдёӢдёҖж¬ЎеҢ–еӯҰжІ»з–—еүҚдёҖдёӘжҳҹжңҹиҝӣиЎҢпјӣеҝ«йҖҹд№ҷйҶҜиҖ…жҺҘеҸ—182 mgзҡ„hydralazineгҖҒзј“ж…ўд№ҷйҶҜиҖ…жҺҘеҸ—83 mgзҡ„hydralazineпјҢиҖҢmagnesium valproateзҡ„дҪҝз”ЁеүӮйҮҸзӮә40 mg/kgгҖӮ
гҖҖгҖҖ
гҖҖгҖҖз ”з©¶иҖ…иЎЁзӨәпјҢиҝҷйЎ№з ”з©¶зҡ„и®ҫи®ЎжҳҜзӢ¬зү№зҡ„пјҢеӨ§йғЁеҲҶзҡ„з—…жӮЈеңЁеҢ–еӯҰжІ»з–—дёӢз–ҫз—…д»Қ然иҝӣеұ•пјҢиҝҷж ·зҡ„зҺ°иұЎеҸҜд»Ҙд»Ҙжңү7дҪҚз—…жӮЈеңЁз¬¬дёҖж¬ЎеҢ–еӯҰжІ»з–—йҖұжңҹеүҚпјҢжӯЈеңЁжҺҘеҸ—hydralazineдёҺvalproateзҡ„йӮЈдёӘжҳҹжңҹиҝӣеұ•иЎЁзҺ°пјҢеӣ жӯӨпјҢиҫҫеҲ°жІ»з–—еҸҚеә”дёҺзЁіе®ҡз–ҫз—…еҸҜд»Ҙд»…еҪ’еҠҹж–јиЎЁи§Ӯеҹәеӣ жІ»з–—пјҢеӣ зӮәиҝҷжҳҜиҜ•йӘҢеүҚдёҺи®Ўз”»дёӯз–—зЁӢе”ҜдёҖжңүе·®ејӮзҡ„ең°ж–№гҖӮ
гҖҖгҖҖ
гҖҖгҖҖDuenas-GonzalezеҚҡеЈ«еҗ‘MedscapeиЎЁзӨәпјҢд»–зҡ„еӣўйҳҹд№ҹе·Із»Ҹй’ҲеҜ№д№ізҷҢеҸҠеӯҗе®«йўҲзҷҢжӮЈиҖ…иҝӣиЎҢз ”з©¶пјҢз»“жһңжҳҫзӨәиЎЁи§Ӯеҹәеӣ жІ»з–—иғҪеӨҹеўһеҠ еӨ§зәҰ1000з§ҚдёҺеҺӢжҠ‘иӮҝзҳӨжңүе…іеҹәеӣ зҡ„иЎЁзҺ°пјҢиҝҷж ·зҡ„жҲҗжһңжҳҜеңЁ8еӨ©зҡ„hydralazineдёҺvalproateжІ»з–—еҗҺд»Ҙеҫ®йҳөеҲ—еҲҶжһҗеҺҹеҸ‘жҖ§иӮҝзҳӨиҖҢжқҘпјӣйҷӨжӯӨд№ӢеӨ–пјҢд»–иЎЁзӨәпјҢеҸҰдёҖдёӘжқҘиҮӘзҫҺеӣҪLee MoffittзҷҢз—Үдёӯеҝғзҡ„з ”з©¶еӣўйҳҹпјҢжңҖиҝ‘жҠҘе‘ҠдәҶдёҖйЎ№й’ҲеҜ№иҝҮеҺ»жҺҘеҸ—йҮҚеәҰжІ»з–—зҡ„еӣәдҪ“иӮҝзҳӨжӮЈиҖ…пјҢеңЁepirubicinжІ»з–—д№ӢеүҚдҪҝз”Ёvalproic acidзҡ„з ”з©¶пјҲMunster PзӯүдәәпјҢJ Clin Oncol 2007;25:1979-1985пјүпјӣ他们жҠҘе‘Ҡ9дҪҚпјҲ22%пјүзҪ№жӮЈдёҚеҗҢзҷҢз—Үзҡ„жӮЈиҖ…жңүйғЁд»ҪеҸҚеә”гҖҒ16дҪҚжӮЈиҖ…иҫҫеҲ°з–ҫз—…зЁіе®ҡжҲ–дәӣеҫ®еҸҚеә”пјҲ39%пјүпјҢиҷҪ然иҝҷйЎ№з ”з©¶дёӯжңӘдҪҝз”ЁhydralazineдҪңзӮәеҺ»з”ІеҹәеҢ–иҚҜзү©гҖӮ
гҖҖгҖҖ
гҖҖгҖҖDuenas-GonzalezеҚҡеЈ«жҢҮеҮәпјҢеўЁиҘҝе“Ҙеӣўйҳҹзӣ®еүҚжӯЈеңЁиҝӣиЎҢдёӨ项第дёүжңҹдёҙеәҠз ”з©¶пјҢдёҖйЎ№жҳҜй’ҲеҜ№143дҪҚжҢҒз»ӯжҖ§гҖҒеҶҚеҸ‘жҖ§гҖҒжҲ–иҪ¬з§»жҖ§еӯҗе®«йўҲзҷҢз—Үз—…жӮЈпјҢе°Ҷе…¶йҡҸжңәеҲҶжҙҫжҺҘеҸ—cisplatinдёҺtopotecanпјҢеҠ дёҠжҲ–жңӘеҠ дёҠhydralazineдёҺvalproateпјӣеҸҰеӨ–дёҖйЎ№е°Ҷдјҡй’ҲеҜ№211дҪҚеҜ№cisplatinз”ўз”ҹжҠ—иҚҜжҖ§зҡ„еҚөе·ўзҷҢжӮЈиҖ…пјҢе°Ҷе…¶йҡҸжңәеҲҶжҙҫеҲ°д»…жҺҘеҸ—topotecanжҲ–еҠ дёҠhydralazineеҸҠvalproateпјӣеңЁиҝҷдёӨйЎ№з ”з©¶дёӯпјҢиҜ•йӘҢз»ҲзӮ№йғҪжҳҜз–ҫз—…жңӘиҝӣеұ•зҡ„еӯҳжҙ»зҺҮгҖӮ
Hydralazine and Valproate Appear to Overcome Resistance to Chemotherapy
The combination of 2 commonly used drugs, hydralazine and magnesium valproate, appears to overcome resistance to chemotherapy. A single-cohort phase 2 study, reported in the September issue of the Annals of Oncology, found that the drug combination led to clinical benefit in 12 of 15 patients (80%). These patients had various tumors and were actively progressing despite chemotherapy. After a course of hydralazine and valproate, rechallenge with the same chemotherapy produced partial response in 4 patients and disease stabilization in 8 patients.
The combination is thought to work by reversing epigenetic aberrations that lead to chemotherapy resistance: hydralazine, an early antihypertensive agent, acts as an inhibitor of DNA methylation, while the antiepileptic magnesium valproate is an inhibitor of histone deacetylation.
\"These results are very clearly suggestive that demethylation and histone acetylation somehow erased the epigenetic mark, leading to chemotherapy resistance, as has been shown in a multiplicity of experimental models,\" lead author Alfonso Dueñas-GonzГЎlez, MD, from the Instituto Nacional de CancerologГӯa, in Tlapan, Mexico, tells Medscape. His group is now conducting 2 randomized phase 3 clinical trials of hydralazine and valproate, and he notes that the Mexican health authorities are currently reviewing use of this combination (under the potential trade name Transkrip) for approval in refractory patients with solid tumors. As an oncologist, he says it is worth considering a trial of the combination in refractory patients for whom no other therapeutic option exists and after extensive discussion with the patient. \"This suggestion is based on the very well-known safety of these drugs,\" he comments. However, he adds, \"It is clear that only when phase 3 clinical trials are completed will we know its place in the cancer therapy armamentarium.\"
An accompanying editorial says the results \"are certainly provocative\" but adds that they should be \"interpreted with caution\" because of the heterogeneity of the tumor types and chemotherapy regimens and because patients who showed early progression during the first week of therapy with hydralazine and magnesium valproate were excluded. \"Nevertheless, it is intriguing that reversal of resistance might be possible with a relatively well-tolerated treatment, and this is a concept that is being tested in a number of ongoing trials of epigenetic therapies,\" write the editorialists, Robert Brown, PhD, from Imperial College, in London, United Kingdom, and Rosalind Glasspool, MD, from the Beatson West of Scotland Cancer Center, in Glasgow, Scotland.
Variety of Tumors and Chemotherapy Schedules
For the current phase 2 study, the researchers explain that 27 patients signed informed-consent documents, but 3 were ineligible and did not initiate hydralazine and valproate, while 7 patients abandoned the study during the first week of this therapy, before the first administration of chemotherapy, due to clinical deterioration. A further 2 patients completed 2 chemotherapy cycles in the study protocol but were unevaluable because progression before their inclusion in the protocol could not be confirmed. These 2 patients were included in the 17 that were evaluable for toxicity, but not in the 15 patients evaluable for response.
The range of cancers included ovarian (7 patients), cervix (3), breast (3), lung (1), and testis (1), and chemotherapy schedules included cisplatin, carboplatin, paclitaxel, vinorelbine, gemcitabine, pemetrexed, topotecan, doxorubicin, cyclophosphamide, and anastrozole. All patients had received at least 2 courses of chemotherapy and showed progressive disease at the second or third chemotherapy course. Protocol therapy started within 30 days after progression was confirmed, beginning a week before chemotherapy was restarted. Hydralazine was given at a dose of 182 mg for rapid acetylators and 83 mg for slow acetylators, and magnesium valproate at a dose of 40 mg/kg.
\"This study design is unique in that the majority of patients were actively progressing on chemotherapy,\" say the researchers. \"This is best illustrated by the fact that 7 patients deteriorated clinically during the week of hydralazine and valproate administration and prior to the first chemotherapy cycle. Therefore, the achievement of response and disease stabilization can be attributed only to the use of the epigenetic agents, because this was the only difference between prestudy and protocol regimens.\"
Dr. Dueñas-GonzГЎlez told Medscape that his group has also conducted previous studies in breast cancer and cervical cancer that show that epigenetic drugs are able to upregulate about 1000 genes implicated in tumor suppression, as shown by microarray analysis in the primary tumors after 8 days of hydralazine and valproate therapy. Also, he notes that a separate group of researchers, from the Lee Moffitt Cancer Center in the United States, has recently reported a phase 1/2 study of valproic acid prior to epirubicin in heavily pretreated solid-tumor patients (MГјnster P et al. J Clin Oncol 2007;25:1979-1985). They reported partial responses across different tumor types in 9 patients (22%) and stable disease/minor responses in 16 patients (39%), although they did not use hydralazine as a demethylating agent.
The Mexican team is now conducting 2 phase 3 trials, Dueñas-GonzГЎlez said. One will involve 143 cervical cancer patients with persistent, recurrent, or metastatic disease, randomized to receive cisplatin and topotecan with or without hydralazine and valproate. The other will be conducted in 211 cisplatin-resistant ovarian cancer patients randomized to single-agent topotecan with or without hydralazine and valproate. In both studies, the end point will be progression-free survival. |